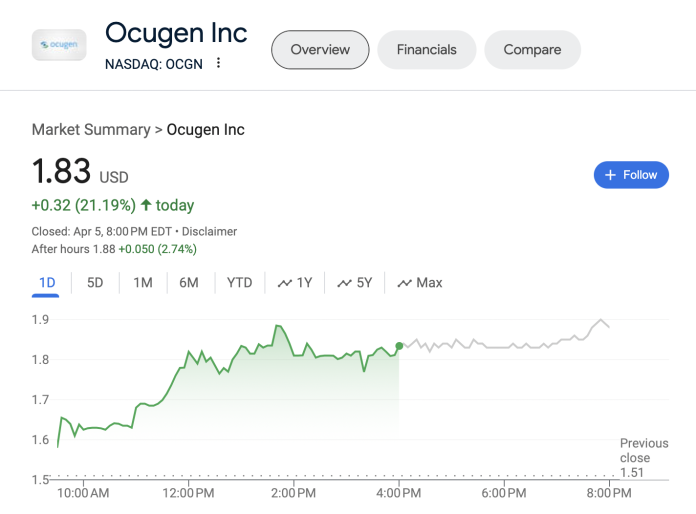
Ocugen, Inc., a pioneer in the field of biotechnology, has recently made a significant stride in the clinical trial of its novel gene therapy, OCU410, aimed at treating Geographic Atrophy (GA), an advanced form of dry age-related macular degeneration (dAMD). This development heralds a potential breakthrough in a market that is eagerly looking for more effective treatments.
Exciting Advances in Treatment for Geographic Atrophy: Ocugen’s Latest Clinical Trial DevelopmentsOcugen, Inc., a pioneer in the field of biotechnology, has recently made a significant stride in the clinical trial of its novel gene therapy, OCU410, aimed at treating Geographic Atrophy (GA), an advanced form of dry age-related macular degeneration (dAMD). This development heralds a potential breakthrough in a market that is eagerly looking for more effective treatments.
The ArMaDa Clinical Trial: A New Hope
The Phase 1/2 ArMaDa clinical trial of OCU410 has reached a crucial milestone. The Data and Safety Monitoring Board (DSMB) has given the green light to continue with the medium dose in the dose-escalation phase of the study. This decision comes after the initial dosing of three patients with GA showed no serious adverse events (SAEs), confirming the therapy’s favorable safety profile to date.
What Makes OCU410 Stand Out?
Currently available treatments for GA involve multiple injections per year and typically target only one disease pathway. In contrast, OCU410 is designed to regulate multiple pathways implicated in the disease, such as lipid metabolism, inflammation, oxidative stress, and the membrane attack complex. This comprehensive approach could position OCU410 as a highly effective, one-time treatment for life, administered through a single sub-retinal injection.
Potential Market Impact
Investors and traders should watch Ocugen closely. The company’s innovative approach to treating GA—not just as a symptom but at its multifaceted root causes—could set a new standard in the field. The financial implications of introducing a one-time, comprehensive treatment are substantial. With over 1 million people affected by GA in the U.S. alone and a larger potential market for dAMD treatments, Ocugen’s OCU410 could capture significant market share from the current treatments that offer less convenience and comprehensiveness.
A Look Ahead
The ArMaDa trial will proceed with further dosing, and a randomized, outcome-assessor-blinded Phase 2 study will follow to solidify the dosage and efficacy findings. This step is pivotal as it will provide the robust data needed to push for regulatory approval.
Conclusion
Ocugen‘s progress with OCU410 is a beacon of hope not only for patients with GA but also for investors looking for promising opportunities in the biotechnology sector. The therapy’s potential to simplify and enhance GA treatment could make Ocugen a key player in the ophthalmology market. As the trial progresses, keeping a close eye on Ocugen’s updates will be crucial for anyone involved in the healthcare investment sphere. Stay tuned for what may be a revolutionary change in how we treat retinal diseases.
Source: https://finance.yahoo.com/news/ocugen-announces-positive-data-safety-110200493.html