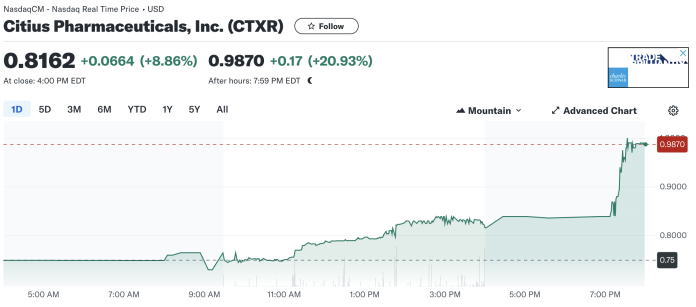
Citius Pharmaceuticals, Inc. (NASDAQ: CTXR) saw its stock price increase by 8.86% to close at $0.8162 at 4:00 PM EDT. After hours, the stock continued to climb, reaching $0.9870, which represents a 20.93% increase from the previous close of $0.7498. The stock opened the day at $0.7501. Throughout the day, the stock price fluctuated between a low of $0.7451 and a high of $0.8391. The 52-week range for the stock has been between $0.6000 and $1.6500 suggesting a significant upside potential. Trading volume for the day was 678,140 shares, somewhat below the average volume of 749,000 shares.
Recently, in a significant development in the fight against B-cell lymphomas, Citius Pharmaceuticals, Inc partnered with the University of Minnesota Masonic Cancer Center to expand the scope of an exciting Phase 1 clinical trial. This trial is focused on evaluating LYMPHIR™ (denileukin diftitox) in combination with FDA-approved CAR-T therapies. The expansion involves the addition of a new study site at City of Hope, enhancing the research capabilities and patient reach of this potentially groundbreaking study.
The trial, originally initiated in May 2021, is spearheaded by Dr. Veronika Bachanova of the University of Minnesota and now joined by Dr. Matthew Mei of City of Hope. It aims to explore the synergistic effects of LYMPHIR™ with CAR-T treatments—tisagenlecleucel (KYMRIAH®), axicabtagene ciloleucel (YESCARTA®), and lisocabtagene maraleucel (BREYANZI®)—specifically targeting the enhancement of lymphodepletion prior to administering CAR-T cells. This innovative approach uses a targeted immunotoxin against IL-2 receptor-positive regulatory T-cells, potentially improving outcomes for patients with diffuse large B-cell lymphomas who are in their second or third line of therapy.
The study is noteworthy not just for its therapeutic potential but also for its strategic collaboration. By engaging with City of Hope, recognized globally for its comprehensive cancer research and treatment, the trial benefits from an enriched research environment and wider participant base. City of Hope’s designation as one of the 53 National Cancer Institute-designated comprehensive cancer centers underscores its pivotal role in cancer research and treatment, making it an ideal partner in this venture.
Dr. Myron Czuczman, Chief Medical Officer at Citius, expressed optimism about the trial’s expansion, highlighting the potential of LYMPHIR™ to modulate the tumor microenvironment in a way that enhances the efficacy of CAR-T cell therapies. According to Dr. Czuczman, this “first-of-its-kind study” is a critical step toward understanding how transient depletion of T-regs can potentially elevate CAR-T-based anti-tumor activities.
As Citius Pharmaceuticals continues to lead and innovate in the biopharmaceutical sector, its collaborative efforts with institutions like the University of Minnesota and City of Hope exemplify the synergy between academic research and biotechnological advancement. The ongoing research not only paves the way for more effective cancer treatments but also illustrates the dynamic nature of medical research in addressing complex diseases like lymphoma.
For more updates on this promising research and other innovative projects at Citius Pharmaceuticals, stay tuned to their latest developments at Citius Pharma. As this trial progresses, it holds the promise to significantly influence the landscape of lymphoma treatment, potentially offering new hope and better outcomes for patients battling this challenging disease.