Adial Pharmaceuticals, Inc. (NASDAQ: ADIL), a clinical-stage biopharmaceutical company dedicated to developing treatments for addiction and related disorders, has recently made a significant breakthrough in the treatment of Alcohol Use Disorder (AUD). The company announced the publication of a peer-reviewed article that not only underscores AD04’s promising clinical outcomes but also its robust safety profile, particularly concerning liver safety—a paramount concern in AUD treatments.
The Science Behind AD04
The study published in the European Journal of Internal Medicine examines AD04, a low-dose ondansetron, which is now leading the charge as Adial’s investigational new drug. This medication targets individuals with AUD who have a specific 5-marker genetic profile. Crucially, the study reveals that AD04 did not significantly alter key biochemical markers of liver injury such as ALT, AST, and Serum Bilirubin, which are often problematic in AUD patients. Moreover, it highlights that GGT levels, typically elevated due to excessive alcohol consumption, were not adversely affected by the drug.
Safety and Compliance: A Comparative Edge
What sets AD04 apart is its safety and tolerability profile, which appears comparable to a placebo—a rarity in alcohol treatment medications. High compliance rates and minimal dropout among patients further reinforce its potential as a mainstay treatment option. No significant treatment-related adverse events were reported, presenting AD04 as a potentially safer alternative to existing AUD therapies.
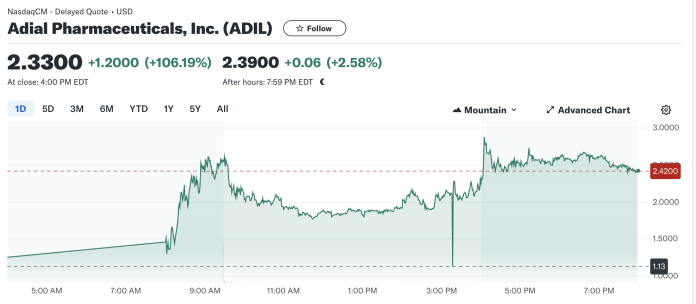
Market Reaction and Trading Data
ADIL’s stock saw a significant uptick, surging over 106% to close at $2.3300, up from the previous close of $1.1300. This surge reflects investor confidence boosted by the promising trial results and the potential market for AD04. The trading volume exploded to 105 million, dwarfing the average, underscoring a heightened investor interest.
CEO’s Vision for AD04
Cary Claiborne, the CEO of Adial, emphasized the need for better pharmacological treatments for AUD, which are often hindered by low efficacy and adverse effects. “This publication validates AD04’s capacity to meet critical needs in AUD treatment with an outstanding safety profile,” Claiborne stated. He also highlighted the broader potential of AD04 to address other addictive disorders, such as opioid use disorder, gambling, and even obesity.
Looking Ahead: Strategic Implications for Investors
AD04’s trajectory represents a promising investment opportunity, particularly as Adial moves closer to potentially securing FDA approval. For investors, the appeal lies in Adial’s strategic positioning within the biopharmaceutical sector, focusing on precision treatment modalities that could revolutionize how addictive disorders are treated globally.
Investors and traders should watch for further developments from Adial’s ongoing research and potential partnerships that could expand AD04’s reach and efficacy. As Adial continues to innovate at the intersection of genetic targeting and addiction treatment, AD04 may well become a cornerstone in combating AUD and enhancing public health outcomes.
For more details on AD04 and to access the peer-reviewed study, visit the European Journal of Internal Medicine’s website.
This article is for informational purposes only and does not constitute financial advice. Investors should conduct their own research or consult with a financial advisor before making investment decisions in the biopharmaceutical sector.